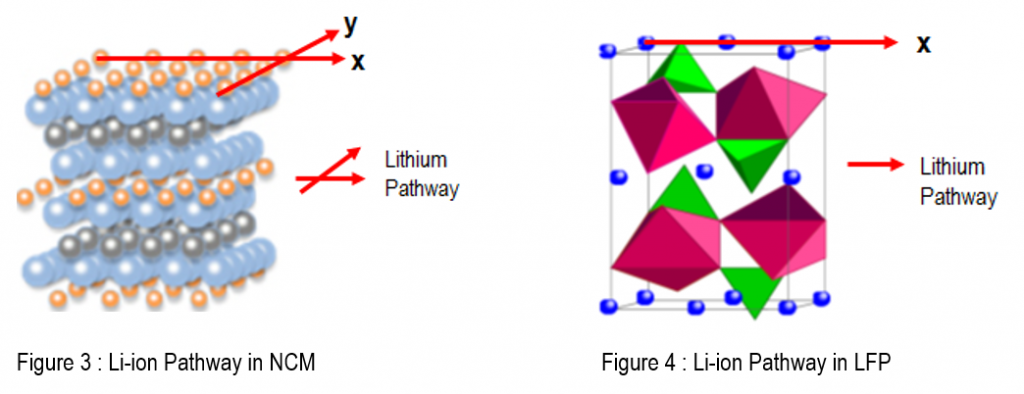
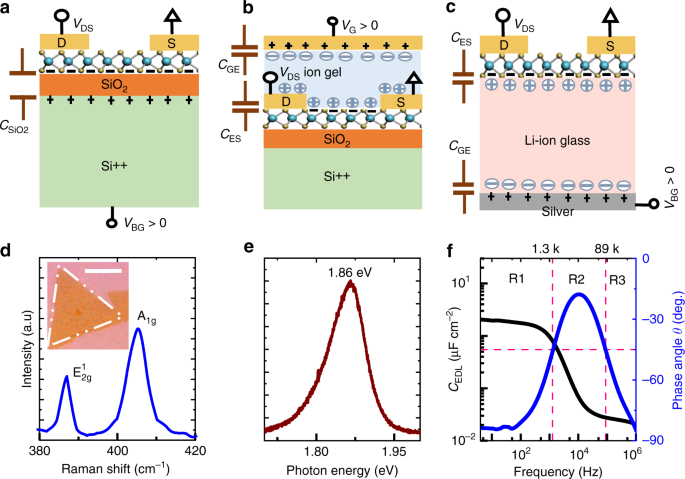
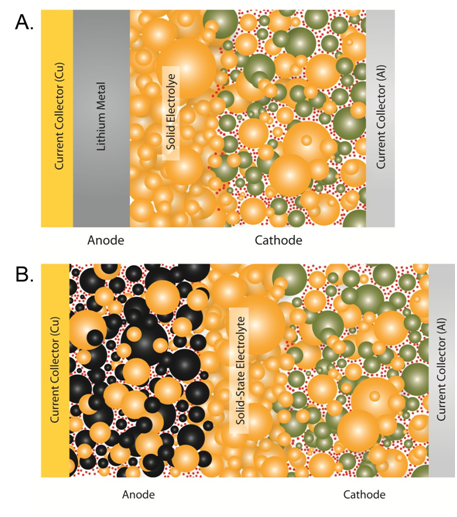
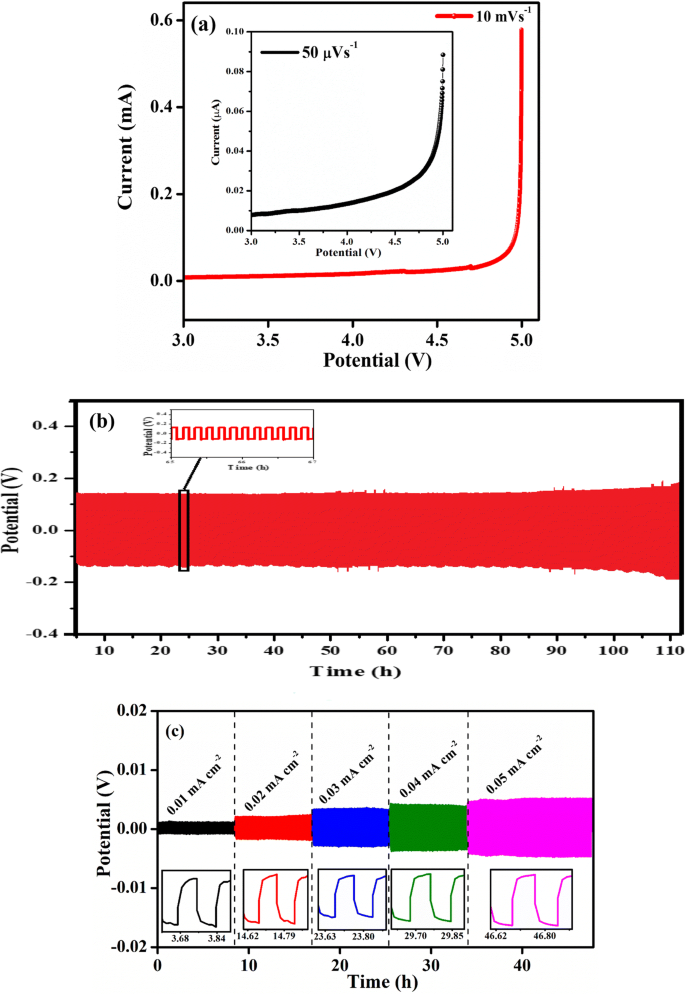
Lithium Conductivity In Ceramics
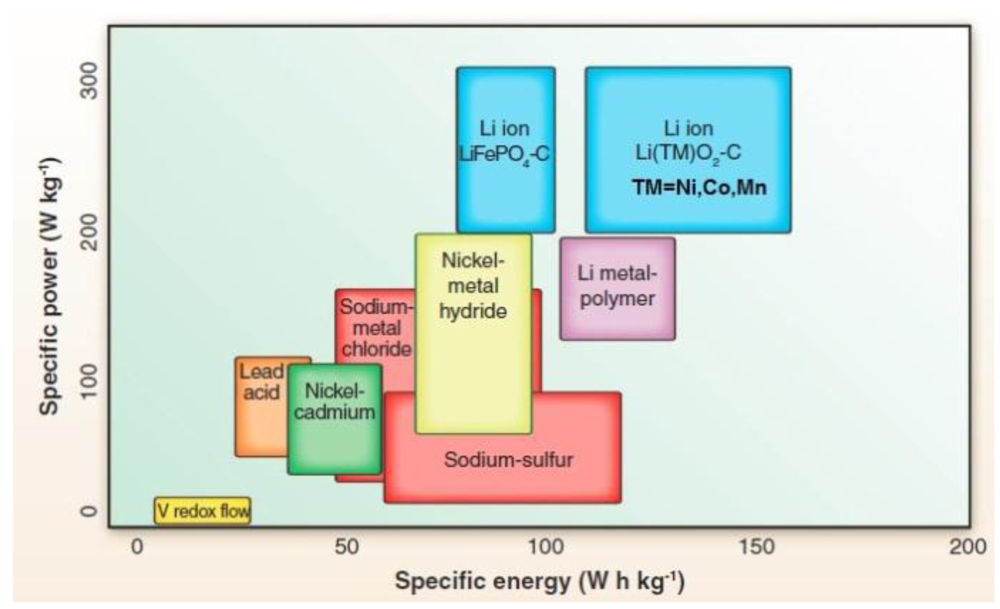
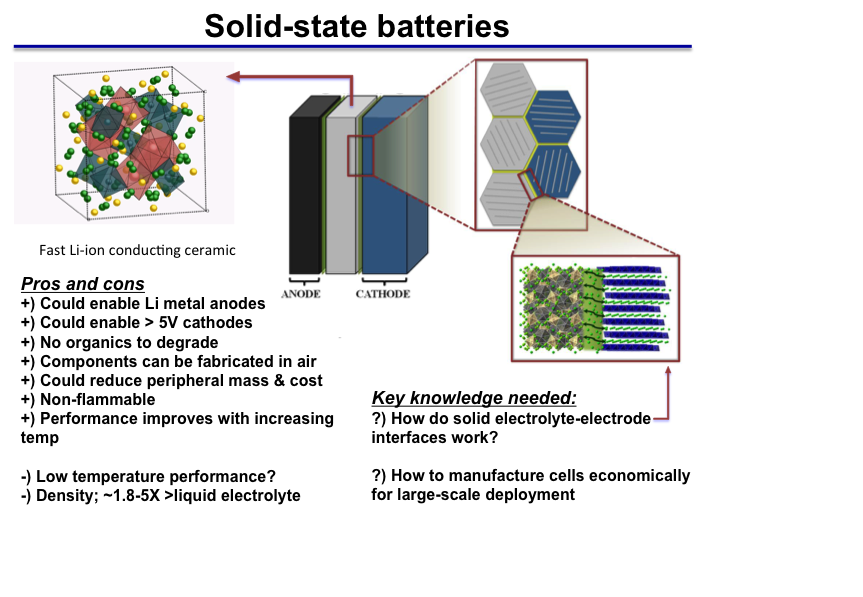
However the combination of both high mechanical properties and high lithium conductivity is still challenging for spes.
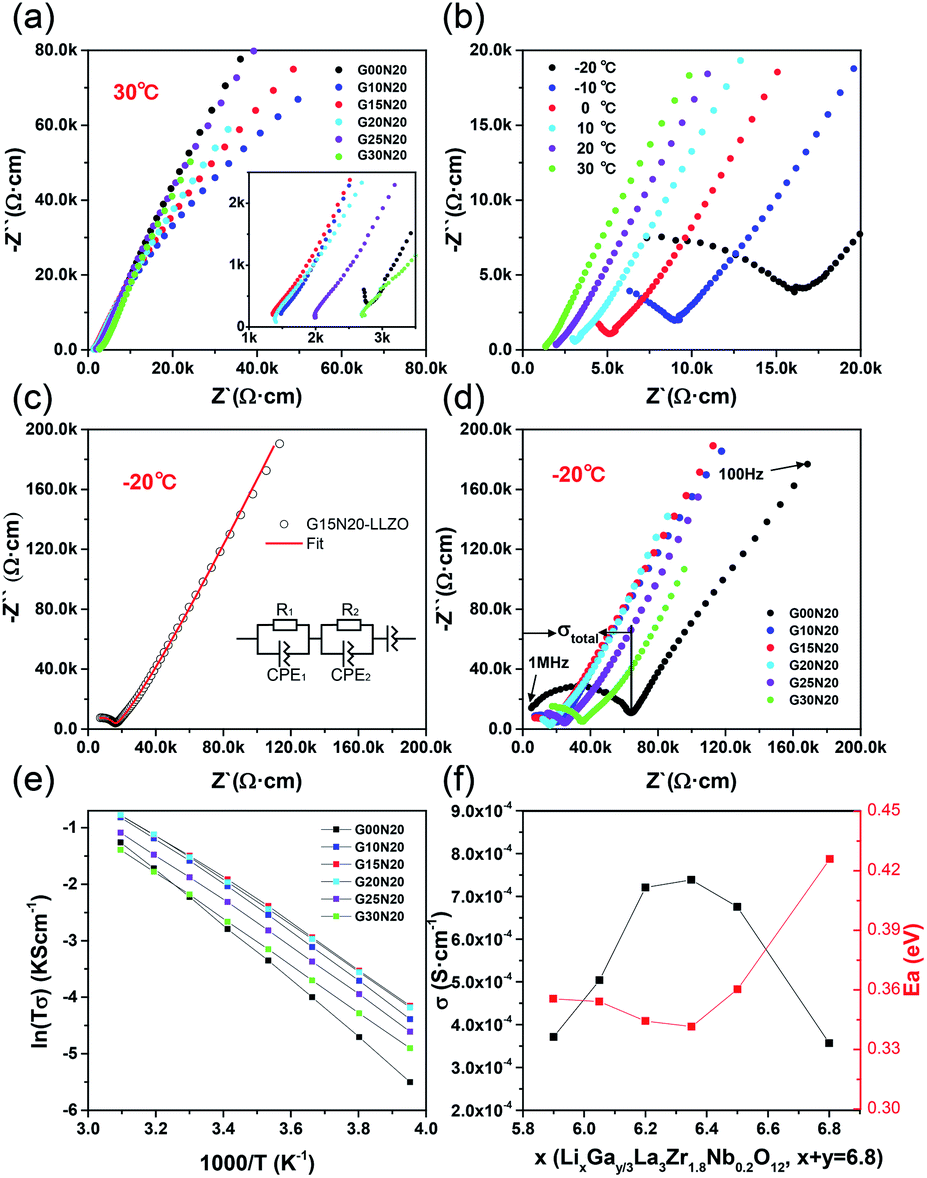
Lithium conductivity in ceramics. Abstract lithium zirconium phosphate lizr2p3o12 thin films have been prepared on platinized silicon substrates via a chemical solution deposition approach with processing temperatures between 700. Jacers is a leading source for top quality basic science research and modeling spanning the diverse field of ceramic and glass materials science. The bulk ion conductivity σdc improves for the specimen with 0 75 mol nd. The highest conductivity of the lagp glass ceramic material at was obtained by crystallizing the glass sheet at for specimen 2.
Although the composites with ceramics such as sio 2 and al 2 o 3 have significantly increased lithium diffusion. At 298 k the ionic conductivity of densified li 1 2 x ca x si 2 n 3 x 0 075 ceramic reached 1 6 10 5 s m 1 almost four orders of magnitude higher than that of densified li 1 2 x ca x si 2 n 3 x 0 ceramic 3 1 10 9 s m 1. Licgc has excellent chemical resistance. 1 to 4 x 10 4 s cm at room temperature.
Water and mild acid have minimal influence on the lithium ion conductivity. Lithium ion transport is 1. For geo 2 free glass ceramics a slight increase in the electrical conductivity is evidenced whereas a conductivity decrease for glass ceramics containing up to 20 mol geo 2 is related to the reduction of a number of lithium ions in the residual glassy phase since the lipo 3 crystalline phase is formed. Among the investigated materials the tantalum compound li 5 la 3 ta 2 o 12 is stable against reaction.
Sulfide compounds in crystalline amorphous and partially crystalline forms have been used as lithium ion conductors. The observed conductivity is about an order of magnitude higher than reported values 5 7 which can be attributed to the large grain size high density and negligible grain boundary resistance as evidenced in. One example is a li 2 s p 2 s 5 glass or glass ceramic the maximum conductivity for which occurs at 20 30 p 2 s 5 depending on the degree of crystallization the conductivities of some li 2 s p 2 s 5 electrolytes are shown in fig. The activation energies for ionic conductivity 300 c are 0 43 and 0 56 ev for li 5 la 3 nb 2 o 12 and li 5 la 3 ta 2 o 12 respectively which are comparable to those of other solid lithium conductors such as lisicon li 14 znge 4 o 16.
The lithium ion conductivity of the glass ceramics increased with the growth of the li1 xalxti2 x po4 3 phase and a extremely high value 1 3 10 3s cm 1 was obtained at room temperature.